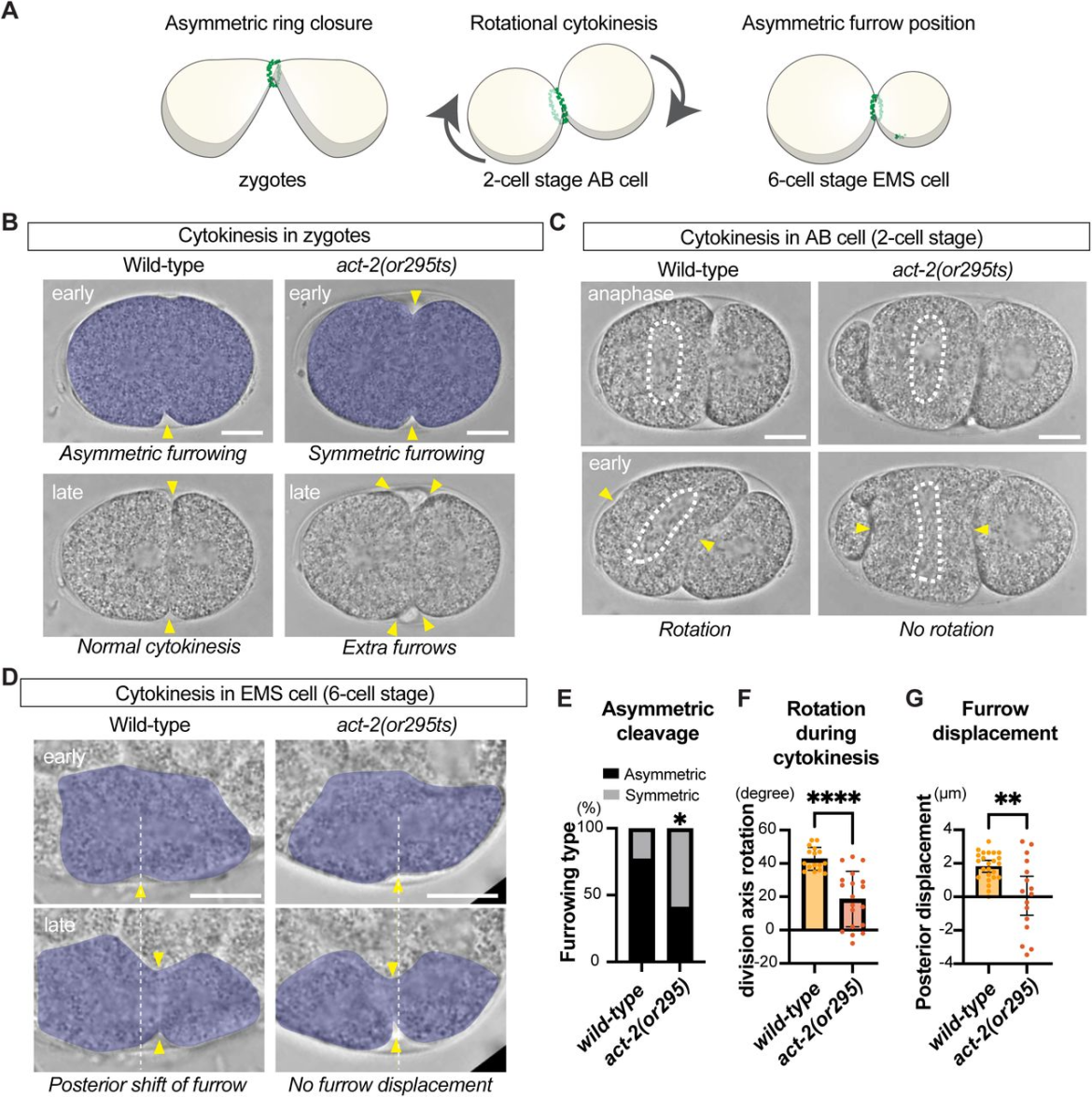
Figure 1. Actin gain-of-function mutant exhibits abnormal spatial control of cytokinesis. (A) Three modes of spatial control of cytokinesis during animal development. (B) Asymmetric contractile ring closure in zygotes. The contractile ring closes asymmetrically in wild-type embryos but is defective in act-2(or295). act-2(or295) mutants also exhibit extra furrow phenotypes at later stage of cytokinesis. (C) Rotational cytokinesis in the AB cell. At the two-cell stage, the AB cell normally rotates during cytokinesis, but this rotation is defective in act-2(or295). Dotted white lines indicate mitotic spindle position. (D) Asymmetric positioning of the contractile ring in the EMS cell. At the 6-cell stage, the EMS forms a cleavage furrow in the equatorial region, which then shifts towards the posterior. In act-2(or295), this posterior furrow shift is often defective. (E-G) Quantification of zygote furrow asymmetry (E), cellular rotation during AB cell division (F), and posterior furrow displacement during EMS cell division (G). Yellow arrowheads indicate the furrow position. Scale bars represent 10 µm. Error bars represent 95% confidence intervals. Fisher’s exact test was performed in panel E and Welch’s t-test was used for panels F and G. P-values: “****”, “**”, “*”and “ns” indicate p < 0.0001, p < 0.01, p < 0.05, and p > 0.05, respectively.
Abstract
Cytokinesis is the final step of cell division, in which the dividing cell is physically separated into two daughter cells by the contractile ring. The contractile ring is a highly resilient molecular machine that can function properly under mechanical stress. Additionally, its function, position, and orientation are spatially modulated in developing animals to regulate morphogenesis. Although essential regulators of cytokinesis have been identified through previous genetic screens, the molecular mechanisms underlying these spatial controls and the mechanical resilience of cytokinesis remain elusive. To identify cytokinesis regulators involved in these processes, we performed a high-throughput RNAi screen using a gain-of-function mutant of actin that exhibits ectopic cortical contraction and abnormal spatial control of cytokinesis in Caenorhabditis elegans embryos. We obtained a total of 483 early embryonic genes that suppress embryonic lethality in an act-2 mutant background. Two parallel secondary screens of candidate genes were conducted. The first secondary screen in a wild-type background identified 71 candidate genes regulating spatial cytokinesis control—asymmetric ring closure, positioning, and rotation—during early embryogenesis. The second secondary screen in the act-2(or295) background identified four genes required for cytokinesis in this background, including microtubule regulators, evl-20/ARL2, and lpin-1/Lipin1. This study will serve as a useful resource for the development of future hypotheses and provide insights into the precise regulation of cytokinesis in tissues.